Overview
The Lu lab seeks to develop well-defined catalysts for converting abundant small molecules, such as N2 and CO2, into useful chemical feedstocks, such as ammonia and methanol, respectively. A big challenge in developing such catalysts is promoting multi-electron and proton-coupled reactivity. Our general approach is to create and test ligand designs that abet transition metals in transferring the electron and/or proton equivalents needed in small-molecule reduction. Our ligands are ideally designed to quickly build a large family of potential transition-metal catalysts that can be screened for catalytic activity in a rapid and efficient manner.
Our research methods include synthesis, spectroscopy, and theory. We are generally interested in creating, understanding, and exploiting new chemical bonding involving a transition metal center. We explore these themes in our current projects:
Configuring New Bonds Between First-row Transition Metals
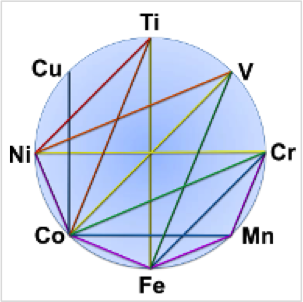
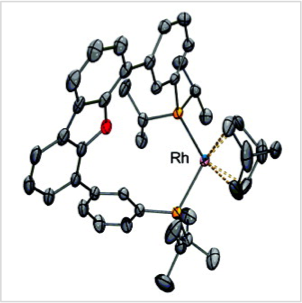
We study M-M and M-M′ pairings of first-row transition metals, ultimately for making cheap and sustainable catalysts. The wider catalytic role of M-M bonds is suggested by the well-known example of Rh-Rh catalysts for functionalizing strong C-H bonds. Bonds between first-row TMs are considerably less known than their heavier analogues, and therefore, ripe for new exploration.
Transition metal pairs can form multiple bonds with versatile, multi-electron redox properties, which is promising for the reduction of small-molecules. A wide range of unique electronic properties and chemical reactivity can be imagined for the vast combinations of first-row TM elements in their various oxidation states. Our research aims to understand how one transition metal influences another, with the ultimate goal of tuning chemical properties and reactivity for the development of highly tailored bimetallic catalysts.
“Deliver-to-Order” Bimetallic Active Sites in Metal-Organic Frameworks (MOFs)
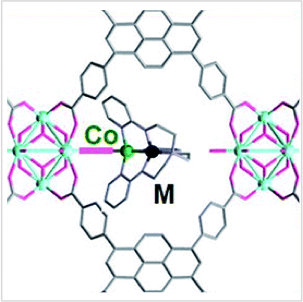
Discovering a catalyst that is perfectly tailored for a specific reaction is like finding a needle in a haystack. To optimize catalyst discovery, we ideally investigate a readily modifiable catalyst system, for which small changes in structure or property can tune reactivity and/or selectivity. Multimetallic clusters are attractive systems to develop because their electronic properties and reactivity should depend on both the number and composition of the individual metals. The key challenge is to assemble uniform and well-defined multimetallic active sites on solid supports. Often, scrambling, agglomeration, and/or other dynamic processes diminish uniformity of metal sites, giving away to complex catalyst speciations that are ill understood.
We have developed the capability to precisely engineer bimetallic active sites on a robust MOF support, NU-1000, where the control over nuclearity and composition are pre-programmed into a bimetallic precursor complex. We demonstrated our synthetic strategy by making uniform bimetallic active sites throughout the MOF material. In on-going work, we demonstrate that reactivity at one metal site can be "promoted" by a proximal second metal. Our dinuclear active sites are highly selective for alkyne hydrogenation to alkenes, whereas the mononuclear analogues only generate alkanes.